- location:
- 網(wǎng)站首頁 >
- PRODUCTS >
- PRODUCTS

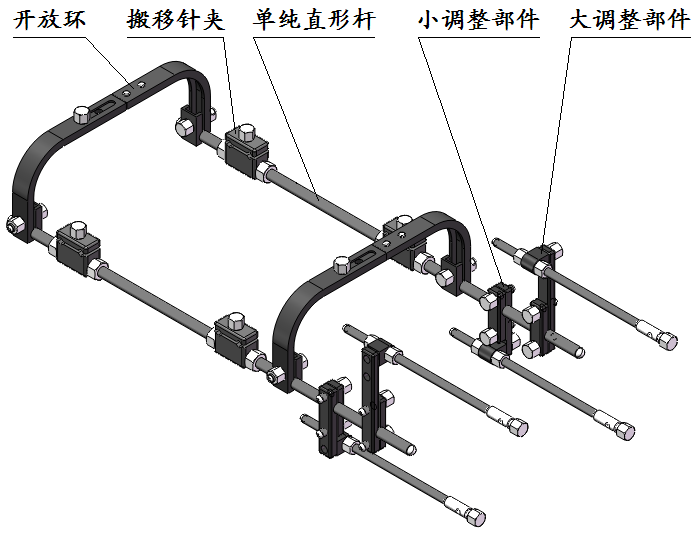




Product structure composition:
Product open ring, moving needle clip, simple straight bar, small adjustment parts, large adjustment parts.
[scope of application]
It mainly adapts to the following symptoms;
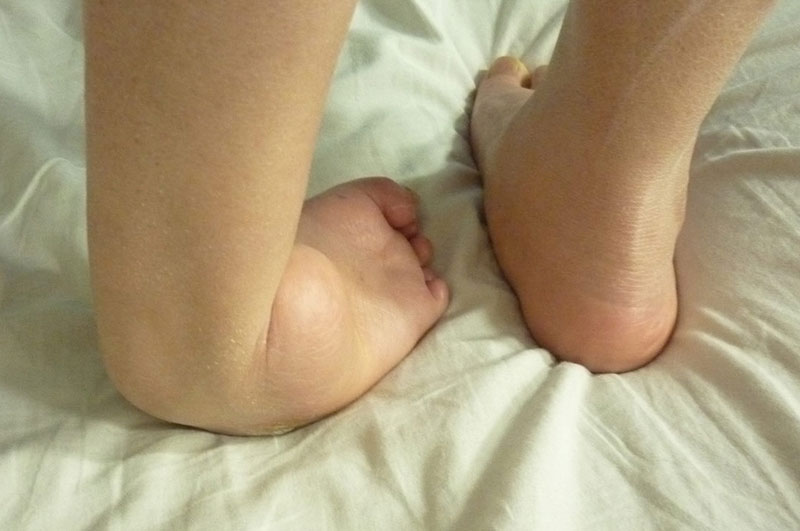
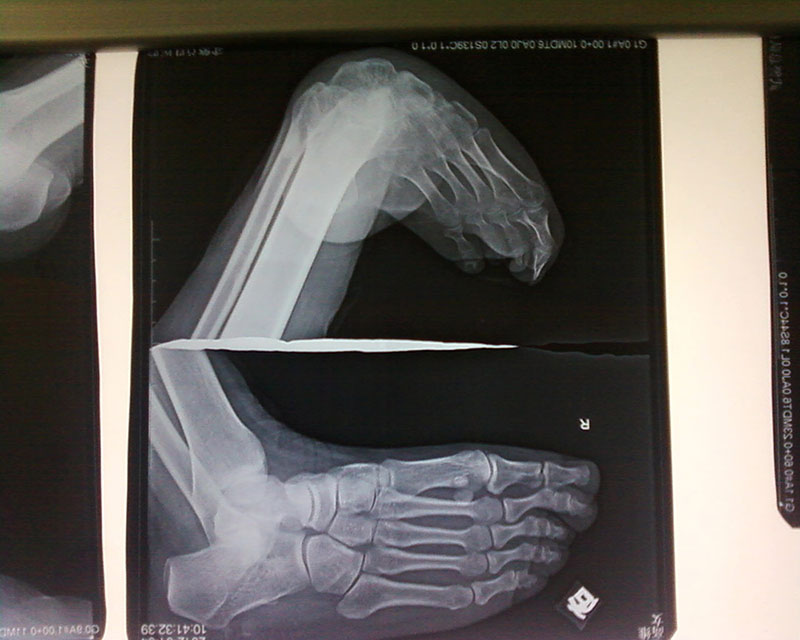
1. Foot drop, foot varus, foot valgus.
2. Foot drop, foot varus and valgus were accompanied with short tibial bone, bone defect and nonunion.
[contraindications] 1. Patients with severe osteoporosis; 2. Patients with extensive skin diseases of injured limbs; 3. Patients who cannot cooperate with postoperative management.
[usage]
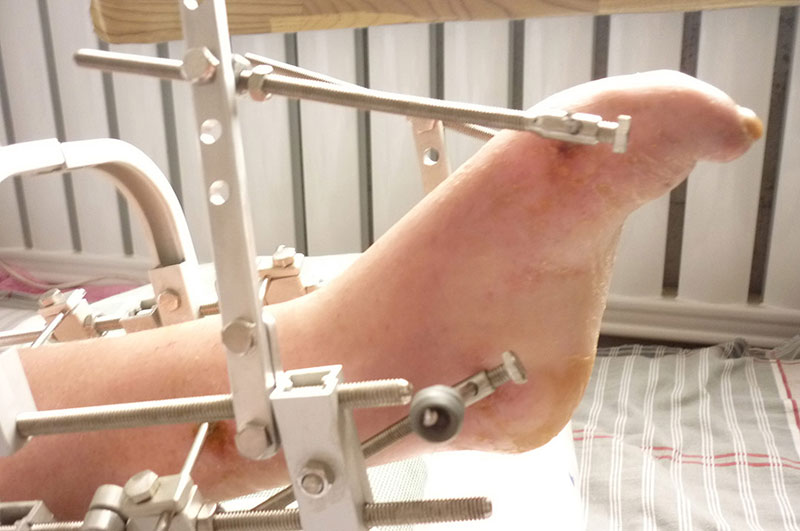
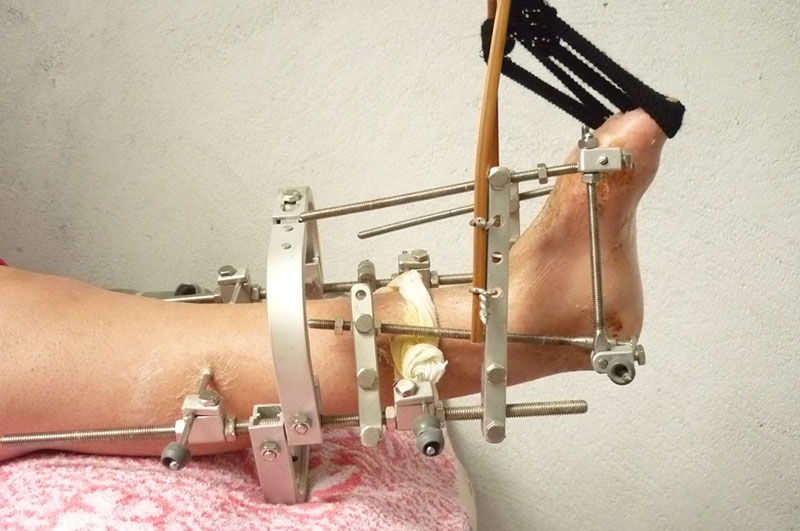
1. According to the medical plan, the position of the orthopedic device was determined, and two bone round needles with a diameter of 4 mm were inserted into the tibia to fix the orthopedic device.
2. A 4 mm diameter bone needle was inserted into the calcaneus and metatarsals respectively.
3. Install the supports and adjustment rods, adjust the position, lock the needle and fasten the components.
4. According to the doctor's guidance to adjust the orthopedic.
[precautions]
1. Use the matching bone needle.
2. The product is not allowed to be reused; it belongs to disposable product.
3. Improper operation shall be strictly prevented.
4. Doctors must be trained and skilled in operation.
5. The appearance and matching performance of the product should be checked before use, and it can be used only after it is qualified.
6. It should be sterilized by high temperature before use.